Regulatory Science of Medical Devices
Evaluation of medical devices to demonstrate the efficacy and the safety is the key to realize biomedical technology to use in society. Faster and clearer evaluation can be the competitiveness of the technology. We study the alternative methods to replace animal experiments and human subject trials, such as in silico clinical trial, organ models with high biofidelity. Topics on utilizing 3D anatomical models complying to ISO 22926 are discussed in Medical 3D Model Consortium Activities.
RELATED PROJECTS
- Bionic Humanoid Project (JST ImPACT)
- Medical 3D Model Consortium (Japanese site)
- ISO/TC 150/WG 14 “Models of tissues for mechanical testing of implants“
Surgical Robotics
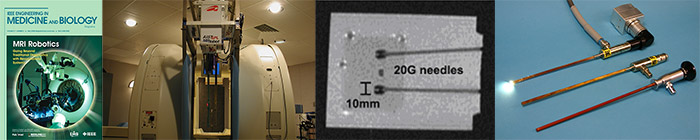
In 1999 we presented world’s first MR compatible robot – robot that works while MR scanner acquires images – and we established MR compatible robotics to design, evaluate the effects between the scanner and the robot.
Current interest is the effective use surgical robot log to develop machine learning for surgical training and autonomous robot control. Another topic is the ELSI (ethical, legal and social issues) on the use of surgical log to balance between ethical considerations and promotion of data use for academic and business purposes.
Chinzei currently serves as the international project leader to develop a safety standard of surgical robots under ISO and IEC.
RELATED ACTIVITY
- ISO/TC 299/JWG5 = IEC/TC 62/SC 62D/JWG 35 “Medical robot for surgery” Project leader
- IEC 80601-2-77:2019
Past works
Biomechanics for Computer Aided Surgery

The brain, liver etc. were not the main interest of classic biomechanics, because the primal functions of these are not mechanical. However, to accurately and precisely control surgical robot or needle biopsy, one needs to predict the deformation of the organs. We started the biomechanics of such organs for surgical technology.
We presented nonlinear constitutive models of brain tissue in the strain and strain rate that practically occur in surgery. We also presented a method to detect the instance of needle insertion using a coaxial needle. Biomechanics will be an essential science for in silico clinical trial and future of autonomous surgical robots.
SCC / Small Computing for Clinicals
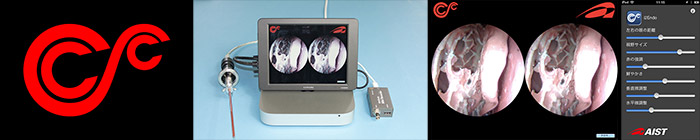
As personal computers (PC) and smartphones evolve, software works as a medical device (SaMD) is attracting market’s focus, since it can disruptively reduce the cost from running on the custom designed hardware.
Small Computing for Clinicals (SCC) is an activity to promote innovative technology and ideas into clinical research for proof of concept.
