Creating Electricity from Waste Heat
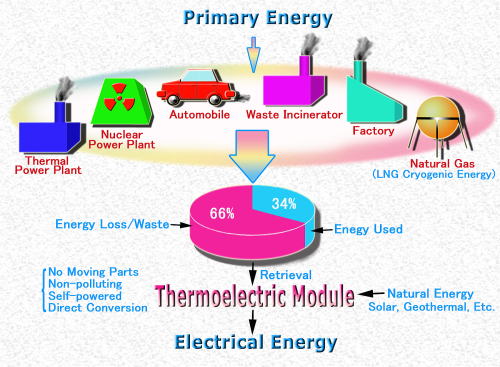
Oil, natural gas, and most other energy sources used in our country, Japan, are imported from overseas. Converted into in petroleum, the amount of energy consumed reaches as much as several 100,000,000 kiloliters per year. We burn these energy sources to produce our electricity, run our automobiles, and even to manufacture chemical fiber products. Effective use of this energy, however, accounts for only around 30%, while the remainder, at around 70%, is wasted as heat energy that escapes into the atmosphere. (See Figure 1.)
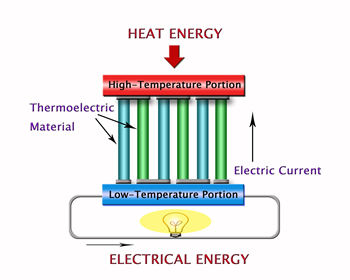
An example of this is the large amount of heat you can feel around the engine of a car. This is caused by the heat energy that is produced and escapes into the air when gasoline is burned. In addition to automobiles, there are a great many other examples of places where waste of heat energy occurs, such as factories and garbage incinerators. In a sense we can also say that naturally generated heat from the Sun and the Earth is also being discarded before efficient use can be made of it. Through recovery and efficient use of such vast amounts of heat energy it should be feasible to limit consumption of dwindling oil reserves as well as the release harmful gases such as CO2 that contribute to atmospheric warming. It is within this context that our group conducts research into thermoelectric systems that directly convert heat energy into electrical energy. As illustrated in Figure 2 thermoelectric conversion is a system whereby electricity is generated simply by applying a temperature difference in a thermoelectric device. Unlike thermal and nuclear systems for power generation, systems such as this produce neither CO2 gas nor radioactive waste.
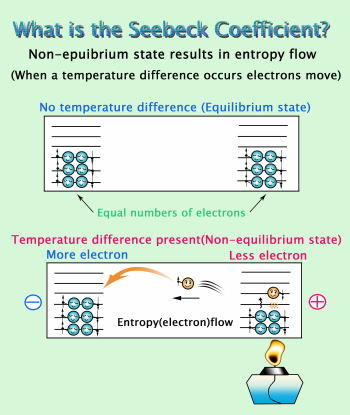
Simply put, they represent an ideal way of generating electricity that is extremely gentle on our precious Earth. Thermoelectric power works on the principle of what is known as the Seebeck effect in which a temperature difference between one side of a thermoelectric material and another produces a voltage (a positive and a negative pole). (See Figure 3).
In simple terms this means that if a small temperature difference can create a large voltage, a large amount of electricity can be generated. By describing the Seebeck coefficient as a voltage that occurs at a temperature difference of 1C° in a thermoelectric material, we can say that the higher the Seebeck coefficient is the better that material is for thermoelectric generation. Thermoelectric materials need to have more than just a large Seebeck coefficient though. They must also allow electricity to pass through easily (low electrical resistivity) and should make it difficult for heat to pass through (low thermal conductivity) so that a large temperature difference may be applied. Moreover, they must also be stable for long periods of use under the high temperature conditions present at incinerators, factories and other places where waste heat occurs. Thermoelectric materials developed so far have been alloys of metals such as bismuth, lead and silver. When exposed to high temperatures in air however, these materials will melt or react with oxygen (oxidization) and thus cannot be used for the purpose of thermoelectric generation from waste heat. It is within this context that our group is now actively developing novel materials with superior thermoelectric properties suited to applications in air at high temperatures.
|