last update 2015.04.23
We have tried to reproduce in Ln, Y and Sc partitioning between Fe-Mn deposit and seawater using simple experimental systems (experimental and theoretical studies of REE in Fe-Mn deposits). In these studies, various irregularities are found in series variation of log Kd data, which may be caused by structural changes of Ln compounds. Lanthanide compounds are not fully isomorphous in the entire series because of the lanthanide effect. For example, Ln3+ aqua ions from La3+ through Nd3+ are nonahydrates, those between Sm3+ and Gd3+ are transitional between nona- and octahydrates, and those from Tb3+ to Lu3+ are octahydrates (Habenschuss and Spedding, J. Chem. Phys. 73, 442-, 1980; Rizkalla and Choppin, Handbook of the Physics and Chemistry of Rare Earths 15, 393-, 1991). The Ln3+(aq) for La-Gd is thermodynamically stabilized in a solution compared to Ln+(aq) for Tb-Lu (Kawabe, Geochem. J. 33, 249-, 1999). As a result, log Kd(REE: FeOOH/solution without NaHCO3) values for light REEs are smaller than those of heavy REEs.
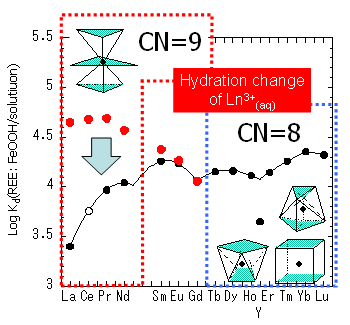
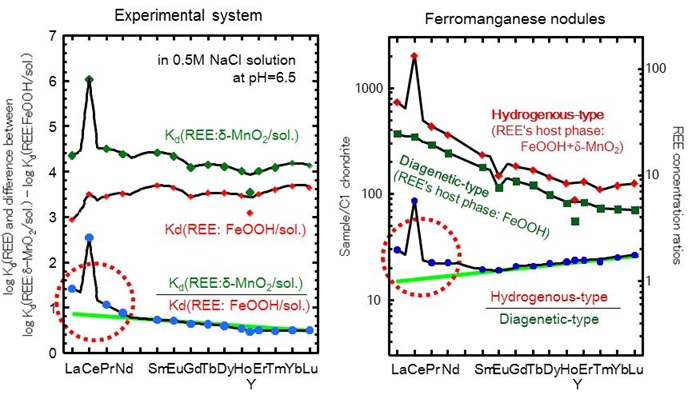
The irregularity described above is also caused by the structural changes in the solid phases. The ratios of log Kd(δ-MnO2/sol.) and log Kd(REE: FeOOH/sol.) have kinked trend in the light REEs. The similar irregularity is also recognized in the field system: the concentration ratio of hydrogenous-type nodule and diagenetic-type nodule. Such irregularity is assumed to be caused by structural changes of REEs in δ-MnO2. However, it is a challenge to determine REE's disorder structures because FeOOH and δ-MnO2 are poorly crystallized materials. In this research, we determined local structures of REE in FeOOH and δ-MnO2 using an extended X-ray absorption fine structure (EXAFS) spectroscopy.
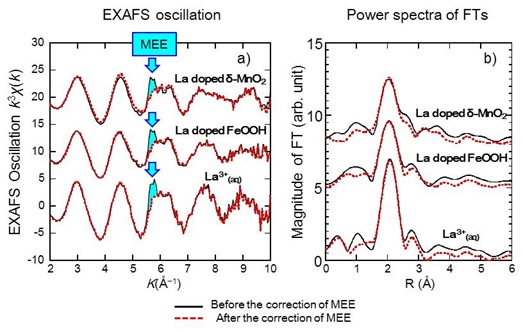
Ln-LIII edge EXAFS analysis has a troublesome problem in the determination of local coordinate structure of lanthanide compounds: the influence of a multi-electron excitation. The sharp absorption of Ln-LIII edge is assigned as 2p → 5d transition (the blue arrow in Figure). The very small peak is superimposed on EXAFS oscillation on the higher energy side of 120-150 eV from the absorption edge. The small peak is caused by a multi-electron excitation and assigned as 2p, 4d → 5d, 5d transitions (Chaboy and Tyson, 1994. Phys. Rev. B, 49, 5869-5875; Chaboy et al., 1994, Phys. Rev. B, 49, 11652-11661). The area of absorption edge of the multi-electron excitation is only less than 1% of 2p-5d absorption edge. However, such excitation engenders a large error in the determination of local coordinate structure of REE, especially for the determination of the coordination number and interatomic distance (Solera et al., 1995. Phys. Rev. B, 51, 2678-2686).
The influence of the multi-electron excitation on the determination of local coordinate structures of REE in aqueous solution or REE in FeOOH and δ-MnO2 were closely examined. We confirmed that significant correction is necessary for the determination of local coordination structure for La in FeOOH and δ-MnO2 but not for those of the other Ln samples. (Ohta et al., 2008. Amer. Mineral., 93, 1384–; Ohta et al., 2009. Amer. Mineral., 94, 467–)
EXAFS analysis has a larger error in determining coordination numbers because the coordination number strongly correlates to the Debye-Waller factor (Ohtaki and Radnai, 1993. Chem. Rev., 93, 1157–1204). Therefore, coordination states of REE in FeOOH and δ-MnO2 are estimated using the interatomic distance (REE-O). The REE-O distance systematically changes as a function of coordination state of REE substance. Three lines in Figure show the mean interatomic (REE-O) distances calculated from various hydrated REE compounds (coordination number (CN) = 8, 9 and 10). The interatomic distances of REE3+(aq) for La, Ce, Pr and Nd are plotted on the CN=9 line, those of Tb-Lu and Y are plotted on the CN=8 line, and aqueous Sm3+, Eu3+ and Gd3+ ions are plotted between the CN=9 and CN=8 lines (blue circle symbols). These results are comparable to Ln3+ hydration sphere reported by previous studies (Habenschuss and Spedding, J. Chem. Phys. 73, 442-, 1980; Rizkalla and Choppin, Handbook of the Physics and Chemistry of Rare Earths 15, 393-, 1991).
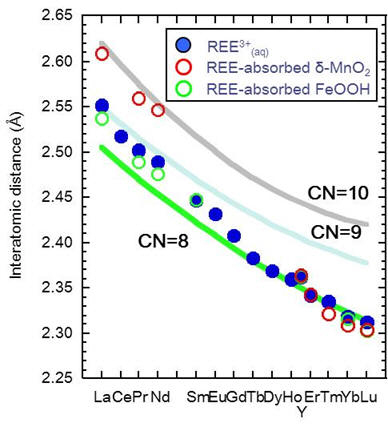
Conclusively, the coordination states of REE3+(aq) and REE in FeOOH and δ-MnO2 are assigned as follows.
REE3+(aq):: La–Nd (CN = 9) ↔ Sm–Gd (CN = 8–9) ↔ Tb–Lu, Y (CN = 8)
REE adsorbed on (or coprecipitated with) FeOOH: La–Sm (CN = 8–9) ↔ Yb–Lu, Y (CN = 8)
REE adsorbed on δ-MnO2: La–Nd (CN = 10) ↔ Er–Lu, Y (CN = 8)
From these data, we concluded that preferential uptakes of La, Pr and Nd by δ-MnO2 are caused by the structural change (Ce is excluded here because Ce is preferentially incorporated into δ-MnO2 by the Ce(III) oxidation process). Incidentally, the Y/Ho fractionation appearing in log Kd data is not caused by such structural changes because the coordination sphere of Y in FeOOH and δ-MnO2 is similar to those HREEs. (Ohta et al., 2009. Amer. Mineral., 94, 467–; Ohta et al., 2009. Amer. Mineral., 94, 476–)
Asakura's Group reported that the full width at half maximum (FWHM) of the white line at the L3-edge XANES for lanthanoid (Ln) compounds can be used as an indicator of the distortion or coordination number of the local environment (Asakura, H. et al., 2014. J. Phys. Chem. C. 118, 20881–; Asakura, H. et al., 2014. Inorg. Chem. 53, 6048–; Asakura, H. et al., 2015. J. Phys. Chem. C. 119, 8070–). Thus, we examine the relationship between the local coordination structure of Ln in Ln3+(aq) and Ln-doped FeOOH, Ln-doped δ-MnO2, Ln-doped calcite, and Ln chemical compounds (Ohta et al., 2018. J. Phys. Chem. A. 122, 8152–). FWHM values of L3-edge XANES of lanthanoid compounds roughly decrease with increasing the coordination numbers of Ln compounds but does not faithfully relate to their local coordination sphere. The FWHM value decreases systematically in the order aqueous ions < Ln-doped FeOOH and MnO2 < Ln-doped calcite <Ln2O3. The FWHM value is rather sensitive to the chemical form of Ln compounds.
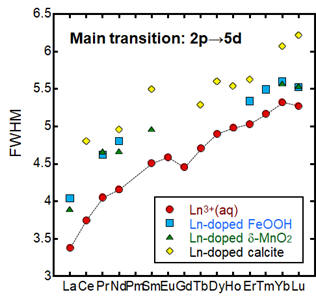
The zigzag pattern appears in the systematic variation of FWHM values across the lanthanoid series: a double-seated-like pattern attributable to 4f electron orbitals. L3-edge XANES corresponds to the excitation of 2p to 5d, nevertheless FWHM for the main transition responds to any effect of 4f electron orbitals. The systematic variation of FWHM can be explained by the following simple equation (Ohta et al., 2018. J. Phys. Chem. A. 122, 8152–):
ΔΓ(q) ≈ Δcfs = Q×{ a + bq + cq2 + dq3 + (9/13)n(S)ΔE1 + m(L)ΔE3 }2
where ΔΓ is a difference between observed FWHM and natural width, Δcfs is the crystal field splitting energy of 5d-orbital, n(S) and m(L) are the constant coefficients given by the quantum numbers S and L, respectively, E1 and E3 shows Racah parameters for the interelectron repulsion between 4f electrons, q is the number of 4f electrons, Q, a, c, b, and d are constants.
The study is described in the research of aeolian dust.
Metal speciation in soil and stream sediments, especially, Cr reduction processes in soil substances. Can naturally-occurring Cr(III) be distinguished from Cr(III) reduced from contaminated Cr(VI) by Fe(II) and organic materials? How do speciation of Cr, Cu, and Zn change in respective depositional environment and weathering process. The study is described in Speciation of metals in soils, stream, lake and marine sediments.