last update 2015.04.23
Chondrite-normalized REE patterns of ferromanganese nodules have light REE enrichment, small negative Eu anomalies, and Y fractionation from Ho. Ce anomalies are positive or negative according to nodule types. Ferromanganese nodules are marine geochemical deposits, nevertheless their REE patterns differ from those of limestone and seawater and rather resemble to average shales such as NASC and PAAS. The Y fractionation from Ho of Fe-Mn nodules, limestone, and seawater indicates that thermodynamic parameters for Y3+ species in marine environments are significantly different from those for Ho3+ even though their ionic radii are almost the same mutually. This is because Y3+ has neither 4f electron nor the Xe core, which is different from Ln3+. [Ohta et al. Geochem. J. 1999, 33, 399-417.; Ohta et al. J. Earth Planet. Sci. Nagoya Univ. 1999, 46, 1-13.]
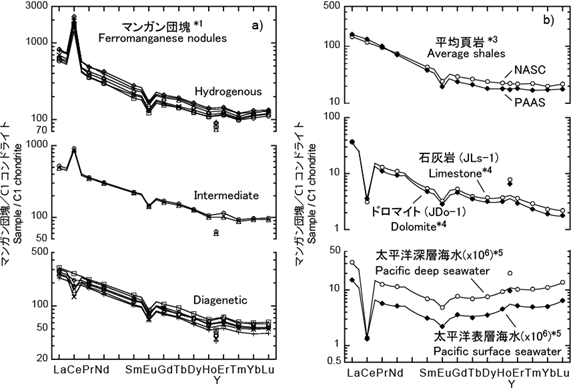
Figure1: Chondrite-normalized REE patterns of various samples. *1 Ohta et al., Geochem. J. 1999, 33, 399-; *2 NASC (Gromet et al., GCA, 1984, 48, 2469-; Haskin et al., Phys. Chem., 1966, 7, 167-) and PAAS (Taylor and McLennan, Handbook on the Physics and Chemistry of Rare Earths, 1998, 11, 485-), *3 Kawabe et al., Geochem. J., 1994, 28, 19-; *4 Moller et al., Chem. Erde: Geochem., 1994, 54, 129- ; Bau et al., Chem. Erde: Geochem.,1995, 55, 1-)
Linearly-related Ce anomaly and Co/(Ni+Cu) ratio is found for Pacific and Antarctic ferromanganese nodules. The systematic correlation indicates how efficiently scavenged-type elements (Co and Ce) have been incorporated into Fe-Mn nodules to nutrient-type elements (Ni, Cu and REE excepting Ce). In addition, three distinct types of Fe-Mn nodules (hydrogenous, diagenetic and suboixc diagenetic) are systematically distinguished in the plot. These facts indicate that an initial common source exists for all the types of nodules. [Ohta et al. Geochem. J. 1999, 33, 399-417.; Ohta et al. J. Earth Planet. Sci. Nagoya Univ. 1999, 46, 1-13.]
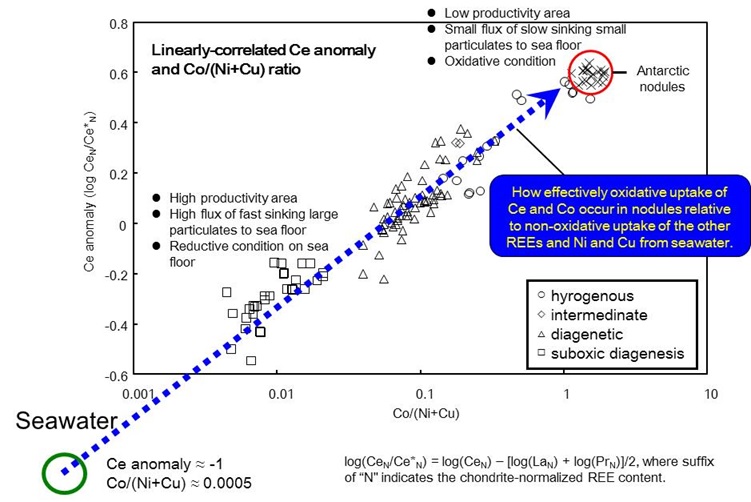
Figure 2: Linearly-related Ce anomaly and Co/(Ni+Cu) ratio in ferromanganese nodules.
REE patterns of ferromanganese deposits normalized by seawater correspond to the apparent distribution coefficients (Kd) of REE precipitation with Fe-Mn deposit from seawater. The Kd(REE: Fe-Mn deposit/seawater) has light REE enrichment, positive Ce anomaly, Y fractionation from Ho, and M-type tetrad effect (blue symbols in Fig. 3). In seawater, dissolved REE species are mainly REECO3+(aq) and REE(CO3)2−(aq) (Byrne and Sholkovitz, Handbook of the Physics and Chemistry of Rare Earths, 1996, 23, 497-). We tried to reproduce these apparent Kd(REE) values using the simple distribution experiment between FeOOH and 0.5M NaCl solutions having HaHCO3 [Ohta and Kawabe, Geochem. J., 2000a, 34, 439-; Ohta and Kawabe, Geochem. J., 2000b, 34, 455-.]
Variation patterns of experimental Kd(REE) between FeOOH and 0.5M NaCl solutions change systematically with increasing NaHCO3 concentrations in the experimental solutions. The ratio of Kd(light REE) to Kd(heavy REE) increases, the fractionation of Y from Ho becomes small, and tetrad effect becomes inconspicuous with increasing NaHCO3 concentration in solutions. Experimental Kd(REE) data obtained in the system with NaHCO3 = 1.3–1.5 mmol/L (CO32−(aq) = 10−4.80–10−4.59 mol/L) mostly duplicate the features of apparent Kd between Fe-Mn deposit and seawater. The carbonate ion concentrations correspond with the value of seawater (CO32−(aq) = 10−4.67–10 −4.30 mol/L).
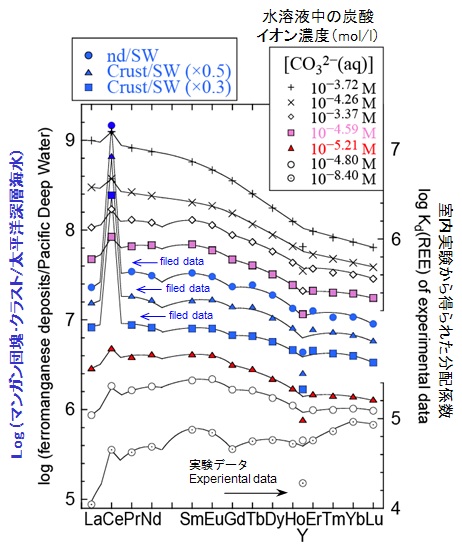
Figure 3: The apparent Kd(REE: Fe-Mn deposit/seawater) and experimental Kd(REE: FeOOH/sol.). Abbreviation of nd, NCP, SCP, and SW indicates Pacific Fe-Mn nodules, North and South Pacific Fe-Mn crusts, and seawater respectively (Ohta and Kawabe, Geochem. J., 2000, 34, 455-).
REE-carbonate ion complexation constants for Ln, Y and Sc are determined from Kd data of REE partitioning between FeOOH and NaCl solution with NaHCO3. The REE-carbonate ion complexation constants have the M-type tetrad effect. The dominant specie of REE in seawater is calculated to be REECO3+(aq) for La-Ho and REE(CO3)2−(aq) for Er-Lu. [Ohta and Kawabe, Geochem. J., 2000a, 34, 439-; Ohta and Kawabe, Geochem. J., 2000b, 34, 455-.]
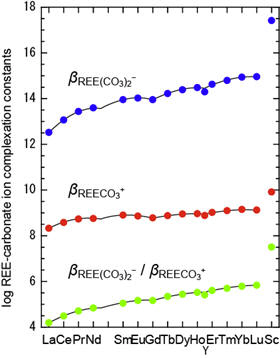
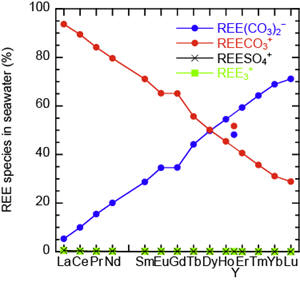
Figure 4: The variations of logarithmic REE-carbonate ion complexation constants and the calculated concentration ratios of REE dissolved species in seawater.
The preferential enrichment of Ce in ferromanganese deposits is caused by the oxidation process of Ce(III) by δ-MnO2 (Goldberg et al., J. Geophys. Res. 1963, 68, 4209-). Actually, experimental REE partitioning coefficients between δ-MnO2 and 0.5M NaCl solution without NaHCO3 shows a distinct positive Ce anomaly. We examined the oxidation state of Mn in δ-MnO2 reacting with Ce(III) and concluded that Ce(III) is oxidized to Ce(IV) by the proton transfer between Ce(III) and Mn(IV) in δ-MnO2. [Ohta and Kawabe., 2000, Geochim. Gosmochim. Acta, 65, 695-703.]
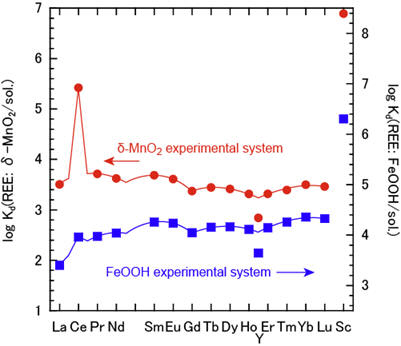
Figure 5: The experimental Kd(REE) partitioning between FeOOH or δ-MnO2 and 0.5M NaCl solution without NaHCO3 in solutions (Ohta and Kawabe., 2000, Geochim. Cosmochim. Acta, 65, 695-703).
Tetrad effects that are found in REE abundance patterns, distribution coefficients, and complexation constants are explained by the Jørgensen-Kawabe equation (Kawabe, Geochem. J. 26, 309-, 1992). The effect reflects the strength of the nephelauxetic effect. The nephelauxetic effect is expressed by the magnitude relation of Racah parameters that are spectroscopically-determined. Racah parameter indicates the inter-electron repulsion of 4f electrons.
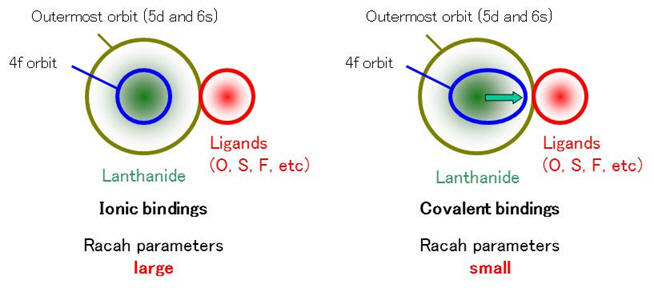
Figure 6: Nephelauxetic effect indicates that 4f orbit of Ln ion extends to the ligand. The inter-electron repulsion of 4f electrons decreases when Ln (relatively) covalently binds with the ligand.
We found that the Racah parameters decrease in the following order (Ohta and Kawabe, Geochem. J., 2000, 34, 455-),
Ln3+(aq) > LnCO3 +(aq)> Ln(CO3)2 −(aq) ≈ Ln(OH)3(ss) (Ln coprecipitated with FeOOH (or δ-MnO2)).
In other words, Ln compounds are relatively covalently-stabilized in the above order. Therefore, tetrad effects found in distribution coefficients and complexation constants are explained as follows.
Incidentally, tetrad effects appearing in NASC-normalized REE patterns of samples are also interpreted roughly as follows,
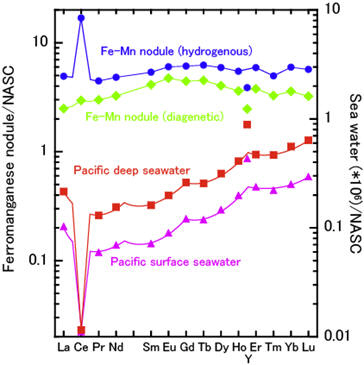
Figure 7: NASC-normalized REE patterns of Fe-Mn nodules and seawater.
NASC-normalized REE patterns of seawater correspond indirectly to the reaction 1. The W-type tetrad effect indicates that Racah parameters of Ln in sea water (mainly LnCO3+: Fig. 4) are larger than those of Ln in NASC. Reaction 2 is previously explained. NASC-normalized REE pattern of Fe-Mn nodule is expressed by the virtual (sequential) reaction 3 (reaction 1+reaction 2). Inconspicuous tetrad effect tetrad effect appearing in NASC-normalized pattern of Fe-Mn nodules is explained by the slight differences of Racah parameters between Ln in NASC and Ln in Fe-Mn nodule. The tetrad effects appearing in chondrite-normalized patterns of various materials are also explained by considering the above successive reactions.