last update 2021.07.28
Cr(VI) has high oxidizing capability and is toxic to the human body. In contrast, Cr(III) is an essential element for living organisms and is both less toxic and less soluble than Cr(VI). The reduction-oxidation process of Cr in natural water and soil has therefore been of great scientific interest. Especially, the Cr(VI) reduction by humic substances in soil environment is important for alleviating environmental problems because the pollutant Cr(VI) can be readily reduced to Cr(III) in field systems. However, little information is available in relation to the chemical state of Cr after Cr(VI) is reacted with soil. Thus, we examine the chemical properties of humic acid after reaction with Cr(VI) and Cr(III) using IR, and the speciation of Cr(VI) and Cr(III) reacting with humic acid, and determine the binding structures of Cr on humic acid using XAFS spectroscopy.
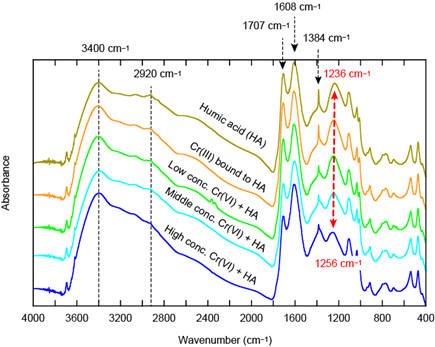
The IR spectra of humic acid reacting with Cr(III) and Cr(VI) show that intensity of IR bands at 3400 cm−1, 1608 cm−1, and 1384 cm−1 increase and that of peaks at 1707 cm−1 and 1236–1250 cm−1 decrease with increasing reacted Cr concentrations. The result suggest the existence of bonding structures of two kinds: hydrated Cr forming an outer-sphere complex with humic acid, and Cr forming an inner-sphere complex with the carboxylate ligand of humic acid (Ohta et al., 2011. Bull. Geol. Surv. Japan. 62, 347–).
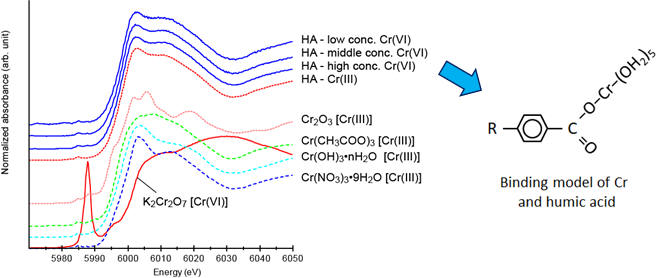
The Cr-K edge XANES spectra of some reference reagents and humic acid reacting with Cr(III) and Cr(VI) (Cr2O72−). The Cr(III) that is reduced from Cr(VI) by HA is mainly bound to HA and partly precipitates as Cr hydroxide (at pH = 4.0–8.0), although unreacted Cr(VI) is remained in experimental solutions (Ohta et al., 2012. Geochem. J. 46, 409–).
Standardization of the sequential extraction procedure and inter-laboratory comparison has been evaluated for the three-step extraction procedure developed by the European Community Bureau of Reference (BCR) (Crosland et al., 1993; Ure et al., 1993). The precision and accuracy of the analytical data using the BCR scheme are confirmed using reference material BCR-701 (lake sediment), which was certified for Cd, Cr, Cu, Ni, Pb, and Zn concentrations for the three steps (Sutherland, 2010). The BCR protocol is applied to sediment geochemical reference materials (lake sediment, stream sediment, marine sediment, and soil) provided by the Geological Survey of Japan, AIST in order to enhance the utility of the sequential extraction procedure in environmental studies. The concentrations of 38 elements (Na, Mg, Al, P, K, Ca, Sc, V, Cr, Mn, Fe, Co, Ni, Cu, Zn, Rb, Sr, Y, Mo, Cd, Cs, Ba, lanthanide (La–Lu), Pb, Bi) extracted by BCR protocols are determined for reference materials (Kubota et al., 2014. Geochem. J. 55, 59–).
We also measure XRD patterns of untreated reference samples and residue of reference samples after sequential extraction. The XRD peaks of calcite, gypsum, and halite in marine sediment samples disappeared after step 1 of the extraction; that of pyrite in inner-bay sediment and stream sediments associated with metalliferous mine is disappeared after step 3. The intensities of XRD peaks of quartz and plagioclase in soil substance increased after the third extraction procedure. It is assumed that organic matter thickly covering the crystalline minerals reduces the reflection intensity from those materials. Nonetheless, BCR protocol does not damage to fragile minerals of clay and mica minerals. (Ohta et al., 2014. Bull. Geol. Surv. Japan 65, 23–)
Brief summary of BCR extraction procedure, regent and extract phase
| Stage | Regent(s) | Reaction time and temperature | Fraction | Target phase(s) |
| Step 1 | CH3COOH (0.11 mol l−1) | 16 hr at 23–25 °C | Acid soluble | Exchangeable phase and carbonate |
| Step 2 | NH2OH HCl (0.5 mol l−1, pH 1.5) | 16 hr at 23–25 °C | Reducible | Fe hydroxide and Mn oxide |
| Step 3 | H2O2 (8.8 mol l−1) (repeated twice) | 1 hr at 20–30 °C; 1 hr at 85 °C; evaporation | Oxidizable | Metal sulfide and organic matter |
| CH3COONH4 (1 mol l−1, pH 2) | 16 hr at 23–25 °C | |||
| Step 4 | HF, HNO3, and HClO4 | 3 hr at 120 °C and then 180 °C for evaporation | Residual | Crystal phase of minerals |
Geochemical reference materials
The toxicity and physical property of metals in sediments and soil substances change according to the chemical species. It is important to know chemical forms of elements in nature in order to distinguish it from a pollutant. A sequential extraction method, a chemical method, has been widely used to speciation study. It has advantage of a simultaneous multi-elemental analysis, high sensitivity detection, and identification many chemical forms. But it might give misleading results because the method cannot dissolve the target phase sufficiently or digest unintended phase. Thus, we apply a x-ray absorption fine structure (XAFS) spectroscopy to identify the metal speciation. If we use plausible reference compounds, we can obtain correct speciation results.
| Method | Features | Detection limit | Multi-component analysis | validity (chemical form) |
| BCR | Decomposition analysis | Several dozen g/kg | 3–6 components | Low |
| XAFS | Non-destructive analysis | Several dozen 10 mg/kg | 2–3 components | High |
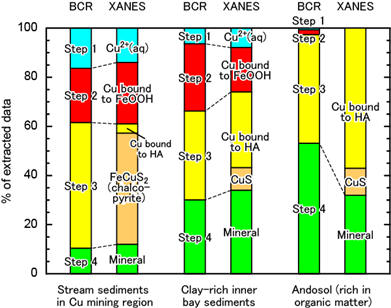
Speciation of Cu in stream sediments and soils are determined using BCR method and XANES method. It is confirmed that the BCR protocol properly extract Cu bound to the "target" phases from sediments (Ohta and Kubota, 2016. Geostand. Geoanal. Res. 40, 117–).
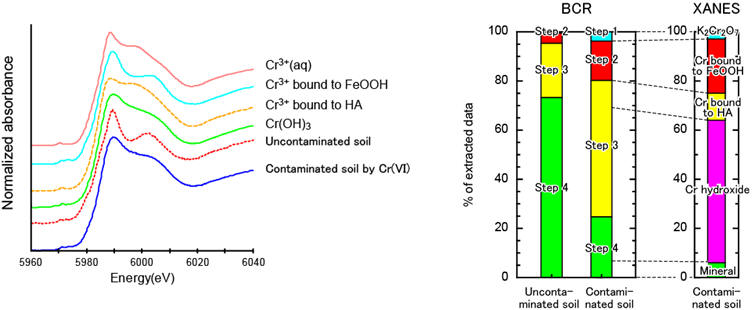
Figure shows the BCR and XANES speciation results of Cr in soil contaminated with Cr(VI). Therefore, Cr(III) resulting from the reduction of contaminated Cr (VI) can be distinguished from naturally occurring Cr(III). The fractions of Cr in reducible phase (step 2) and oxidizable phase (step 3) greatly increased for Cr contaminated sample. The reducible phase (step 2) is identified to be Cr bound to Fe hydroxides by XANES analyses. The Cr extracted in oxidizable phase (step 3) is found to be a mixture of Cr bound to humic acid and Cr hydroxide precipitates. Furthermore, a significant amount of Cr hydroxide is also confirmed to be present in the final residue of materials. Hence, a combination of sequential extraction and XANES spectroscopy is necessary for identifying and quantifying chemical forms in soils (Ohta, 2015. Geostand. Geoanal. Res. 39, 87–).
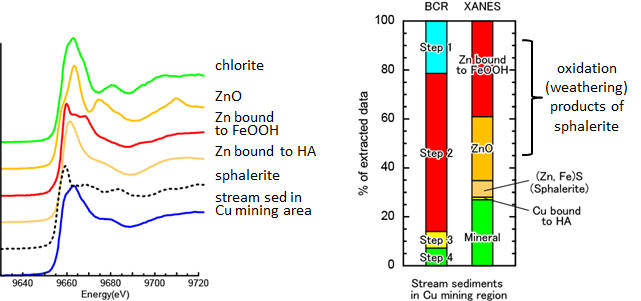
Figure shows the BCR and XANES speciation results of Zn in stream sediments collected from Cu mining region. More than 80% of total Zn is extracted in steps 1 and 2 (BCR protocol). However, XANES fitting results suggest that Zn extracted in step 1 is composed of sphalerite ((Zn, Fe)S), Zn-doped FeOOH, and ZnO; that in step 2 is composed of Zn-doped FeOOH, and chlorite. The presence of Zn-doped FeOOH and ZnO in steps 1 and 2 interpreted as the oxidation products of sphalerite. Furthermore, XANES spectroscopy reveals that significant amount of Zn is extracted from chlorite and micas in sediments. The BCR extraction protocol does not provide the predicted results for Zn speciation, but it provides us crucial information about the potential hazard of Zn in soil, stream sediments, and marine sediments (Ohta et al., 2018. Geochem. J. 52, 385–).